
Electric Vehicle on Fire
It’s no surprise that electronic devices have taken over our daily routines. Not only do we demand the best performance from our phones or laptops, but we demand it everywhere
we go. From hanging out at the beach to taking a hike in the mountains, we take our phones and watches everywhere and we expect them to work. It’s not just portable electronics that are booming right now; almost every major car maker is either currently developing or will develop electric vehicles in some form whether it’s fully electric or a hybrid electric.
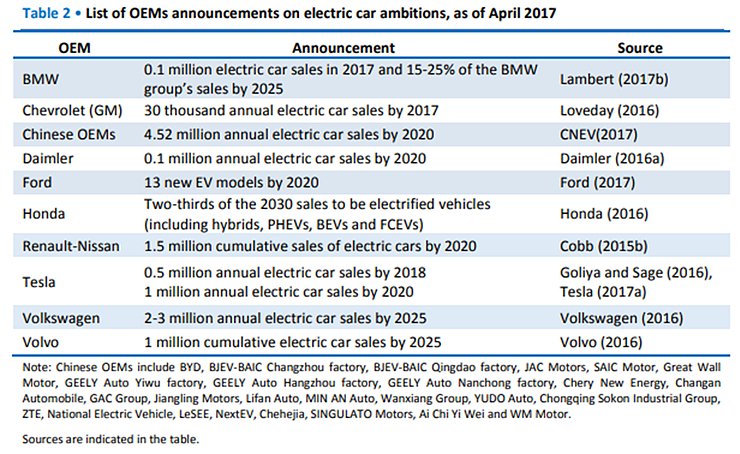
List of OEMs announcements on electric car ambitions, as of April 2017
To get the maximum range out of these electric vehicles, automotive original equipment manufacturers (OEMs) are packing in as many batteries as they can. As electric vehicle performance expectations rise and their designs transition to hold even more powerful batteries, one aspect has struggled to catch up and that is safety.
Current Status of Battery Safety
In the news, we are all familiar with electric vehicles catching fire after a crash, vape pens exploding in people’s pockets, and even incidents with cell phones overheating and igniting into flames. These explosions are usually the result of an internal short circuit caused by damage to the battery (e.g., electric vehicle crash), or even an external and uncontrolled temperature increase (e.g., cell phone battery reaches a high temperature). Unfortunately these incidents are prone to occur in situations we often don’t even think twice about, like leaving your phone out in the sun too long or fast charging. Similarly, for electric vehicles, the fact of the matter is that car crashes are inevitable, so there is a risk that a crash can damage the batteries and cause a short circuit. So if short circuits can happen from common acts or acts out of our control, can we find a way to stop them from happening in the first place?
Why do Batteries Catch Fire?
Before we answer that, let’s dive deeper into the causes of batteries catching fire. During an internal short circuit of a battery, the positive and negative electrode materials are physically and electronically brought into contact, giving rise to high local current densities. A prolonged internal short circuit results in self discharge in combination with a local temperature increase. The high temperatures that result from this can cause the electrolyte to decompose through exothermic reactions, which means the decomposition process itself releases even more heat. If the temperature reaches a certain threshold, a thermal runaway event begins causing more and more heat and energy generation until the flash point of the electrolyte is reached causing ignition.
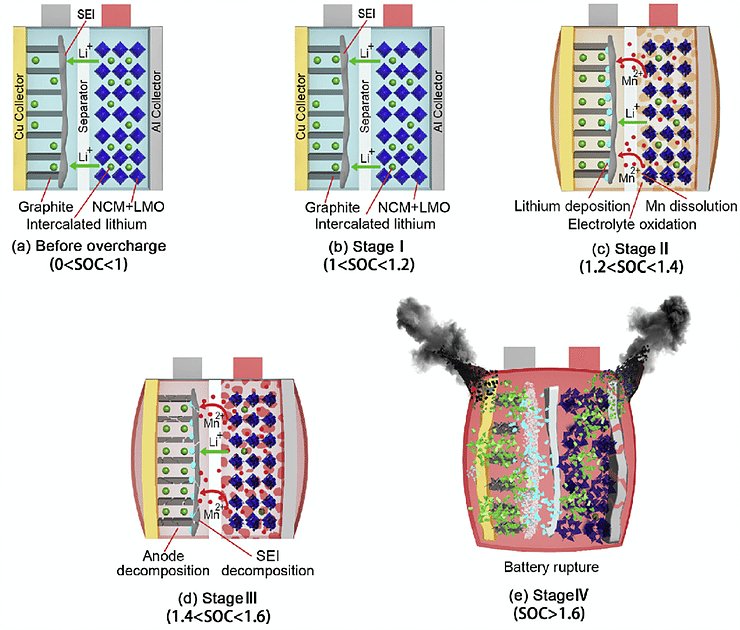
Overview of the overcharge side reactions at each stages for lithium ion batteries with NCM & LMO cathode.
Current Options to Solve the Problem
In industry today, there are a few options to reduce the likelihood of lithium-ion cells from igniting, as well as options to suppress the spreading of fire from cell to cell. One such option is a fireproof casing that holds the many cells together. If an internal short circuit happens in a cell, the runaway reaction is contained in the one cell and does not spread to the dozens of other cells nearby that would create a chain reaction. While this does prevent the ability of the fire from reaching other cells, it does not eliminate the possibility of the cells catching fire in the first place. Another option is attacking the cause of the fires at the source: the battery materials themselves.
The electrolyte is generally the first component of the lithium-ion cells to ignite so this is usually the center of focus in fire prevention. One such method to solve this issue involves the development of a solid-state battery. A solid-state battery is a next-generation battery that uses solid electrodes in tandem with a solid-state electrolyte (SSE) (e.g., ceramics or polymers) instead of the liquid electrolyte found in today’s lithium-ion batteries. While the solid-state battery has a high tolerance against increased temperature and abuse, the SSE it uses is typically expensive to manufacture and inherently weak and inflexible.
Another approach to addressing the flammable electrolyte component is to modify the current liquid electrolyte found in most lithium-ion batteries today. This can be done by changing the chemicals in the solution to raise the inherent temperature at which the chemicals ignite, also known as the flash point. By raising the flash point, the electrolyte is less susceptible to elevated temperatures and prevents the risk of a thermal runaway reaction following a short circuit.
The End Game
The ultimate solution is creating a battery with material components that are inherently non-flammable such as the electrolyte and separator, and pairing them with a casing that localizes any fire that may happen. This combination would give drivers of an electric vehicle the peace of mind that their vehicle will not burst into flames if there is ever a crash or battery overheating.
As battery performance increases so too does the amount of energy they stores which increases the potential for a catastrophic failure if a short circuit were to occur. This calls for a technology that eliminates the possibility of a fire from ever occurring while, ideally, not affecting the battery’s performance. For more information on lithium-ion battery safety, please visit www.theglobalgraphenegroup.com/energy-storage.

