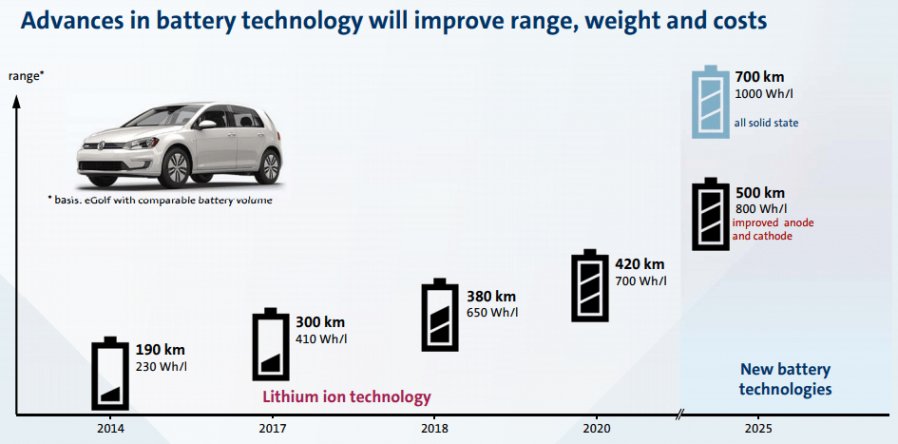
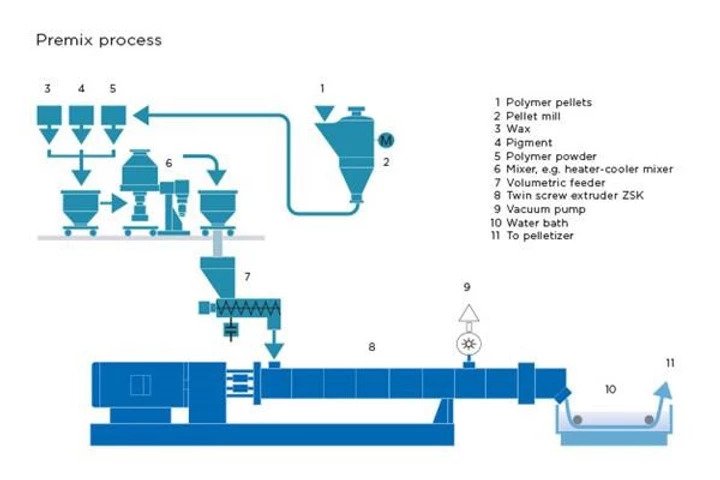
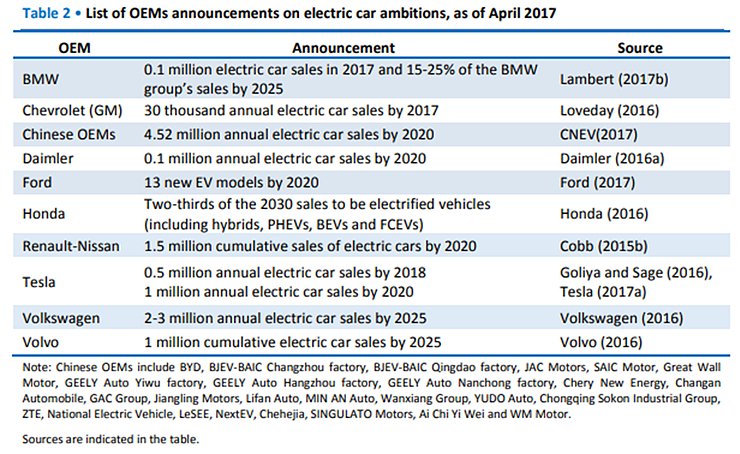
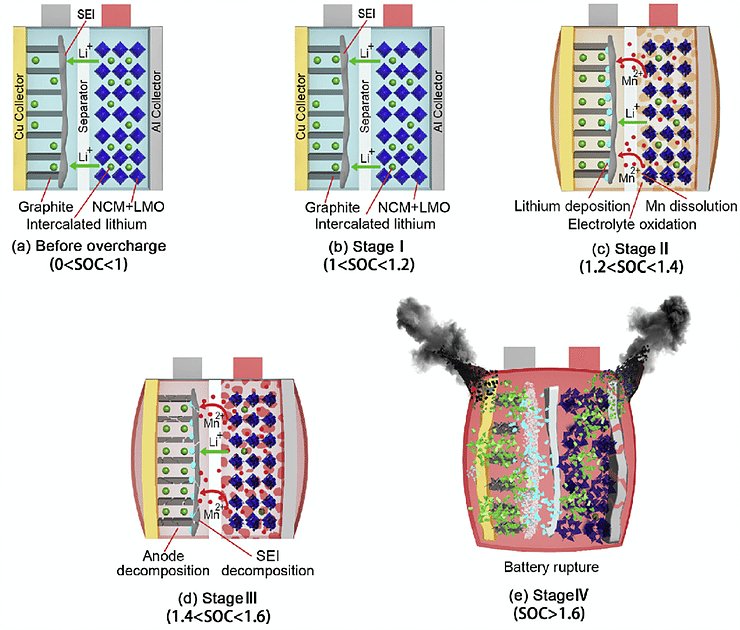
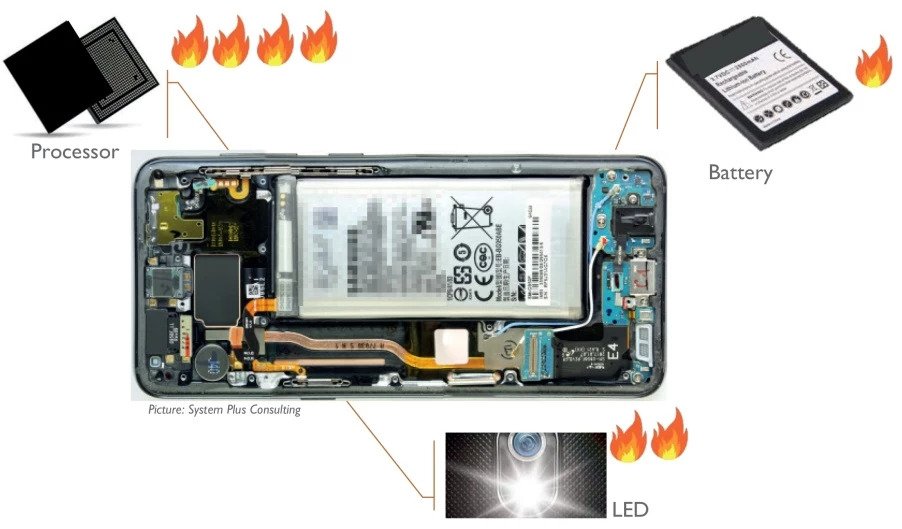
We are all familiar with lithium-ion batteries (LiBs). They power everything from smart phones, laptops, medical devices, electric vehicles and even grid storage. With major companies like CATL, Northvolt, and Tesla currently scaling up their production of LiBs, it is permissible if you’ve forgotten that LiBs are in fact a relatively mature technology. To recap, a typical lithium-ion battery uses carbon (graphite) as an negative electrode and lithium-metal oxide (e.g., NCA, NCM, LCO) as a positive electrode. Using these materials, a LiB’s energy density tops out around 700 Wh/L. Companies are seeking higher energy for space-limited applications, especially electric vehicles, and thus tinkering with the materials inside traditional LiBs to do so. The question bears asking: What then, exactly, are they doing? What comes after LiBs?