Masterbatch In The Plastic Industry
Plastic is one of those materials that we don’t always realize how much it’s involved in our daily lives. From automotive parts to sporting goods, packaging, electronics, and medical devices, we rely on plastics to deliver reliable and quality consumer products. Accordingly, Grand View Research, Inc projects the Global Plastics Market is to reach 654 billion USD by 2020.
What Is Masterbatch And Why Do We Need It?
Before discussion on what a masterbatch is, it is important to note the difference between a polymer and a plastic. A plastic is comprised of a long chain of polymers, whereas polymers are composed of smaller, uniform molecules. Why is this important? Because while all plastics are considered polymers, not all polymers are considered plastics. This knowledge now sets you apart as one of the know-it-alls, and with a proper understanding of this new terminology, we can dive into what a masterbatch is.
Masterbatch a concentrated mixture or pigments and/or additives encapsulated during a heat process into a carrier resign which is then cooled and cut into a granular or pellet shape. This allows for easy processing, storage, consistency and controllability. It can be made from just a simple polymer such as high density polyethylene (HDPE) or something more advanced such as a carbon-fiber composite. No matter the type of masterbatch, they are all made in a similar fashion as seen in the diagram below.
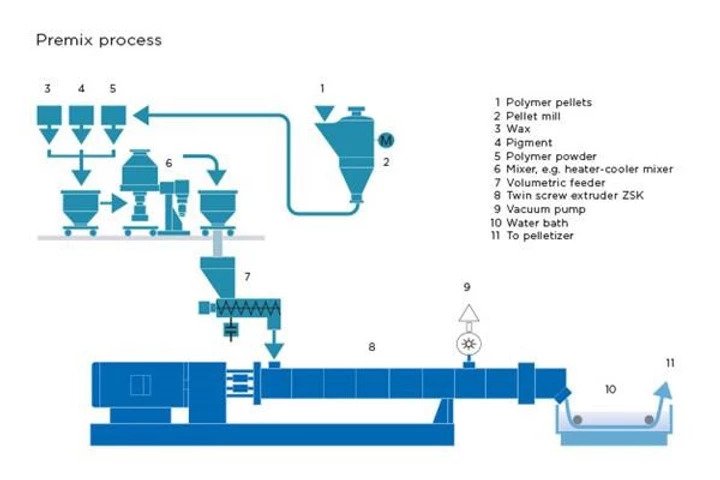
Premix process for manufacturing masterbatch.
Companies will then take the masterbatch, melt it down, and mold it into the end product such as water bottles or packaging. The alternatives to using masterbatch are compounding the materials on site or buying a fully compounded material however both have their own issues. Compounding on site is prone to issues of uneven dispersions and fully compounded material may be more expensive and have less color variety.
Companies looking for improvements from a base plastic masterbatch for higher performance applications, such as sporting goods or automotive parts, will use an enhanced polymer masterbatch that offer improvements in mechanical strength and thermal conductivity. We will cover more about enhanced polymer masterbatches in a later post.
Overall, masterbatch provides companies a convenient way to manufacture polymer or plastic products without requiring extra processing steps or taking up large amounts of storage.
For information on a graphene-enhanced polymer masterbatch, visit www.theglobalgraphenegroup.com/graphene-intermediates